BC3 Technologies
SEAL Hemostatic Wound Spray
The Client
Founded in 2018, the minority-owned company develops groundbreaking, proprietary antihemorrhagic solutions that cater to the medical, military, and general consumer sectors.
With over 200,000 Americans succumbing to trauma-related fatalities each year, BC3’s focus is on saving lives by providing efficient, effective, and accessible hemostatic products, with particular emphasis on their flagship hemostatic aerosol product.

The Challenge
BC3 Technologies received a long-awaited marketing clearance from the U.S. Food and Drug Administration (FDA) for its flagship hemostatic aerosol spray, SEAL, and wanted to use this news to reach key government agencies, medical organizations, and funding sources to begin marketing and selling the product.
However, the company had very little market visibility and were virtually unknown by prospects and investors. Moreover, the company’s website, visual branding, and messaging were outdated, lacked the basic functionality for receiving inquiries, and did not position the company as an authority within its industry.
The Solution
Responsive Website & Media Relations
• • •
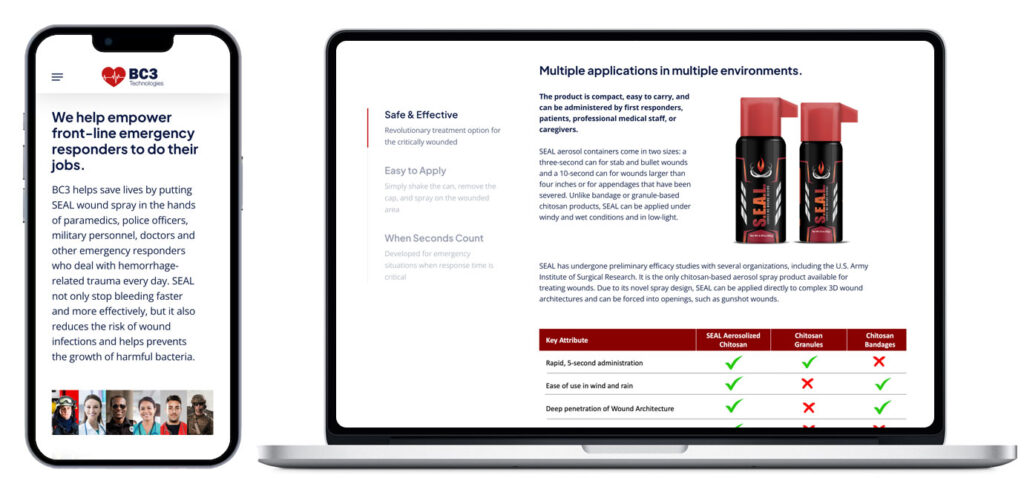
Pugh & Tiller proposed an accelerated website redesign to coincide with the forthcoming FDA announcement and near-term press releases for BC3 Technologies. The aim was to leverage a new website to increase brand-recognition and improve usability and visitor experience ahead of the expected increase in web traffic from media coverage. Pugh & Tiller designed and developed a new mobile-friendly micro-site that appealed to BC3’s visitors in an approachable but sophisticated way, ultimately delivering corporate and any available product information quickly and efficiently. It appealed to prospective investors and immediately conveyed the cutting-edge qualities of BC3. Pugh & Tiller also drafted new company messaging for all external touch points, documents, and content.
Additionally, Pugh & Tiller devised an aggressive but targeted media relations campaign supporting the BC3’s FDA announcement.

The Results
The new website, which was designed to appeal to prospective investors and immediately convey the cutting-edge qualities of BC3, helped to increase brand-recognition, improve usability, and visitor experience. In addition the FDA clearance announcement garnered significant media attention and coverage from local and trade outlets, including the Baltimore Business Journal, Technical.ly Baltimore, World Aerosols Magazine, Spray Magazine, Police Magazine and the editor of Lexipol Media group which consisted of Police1, EMS1, FireRescue1, Gov1, and Corrections1 Magazines. In addition, several organizations posted the news to their own social channels including CityBiz and the Maryland Department of Commerce.